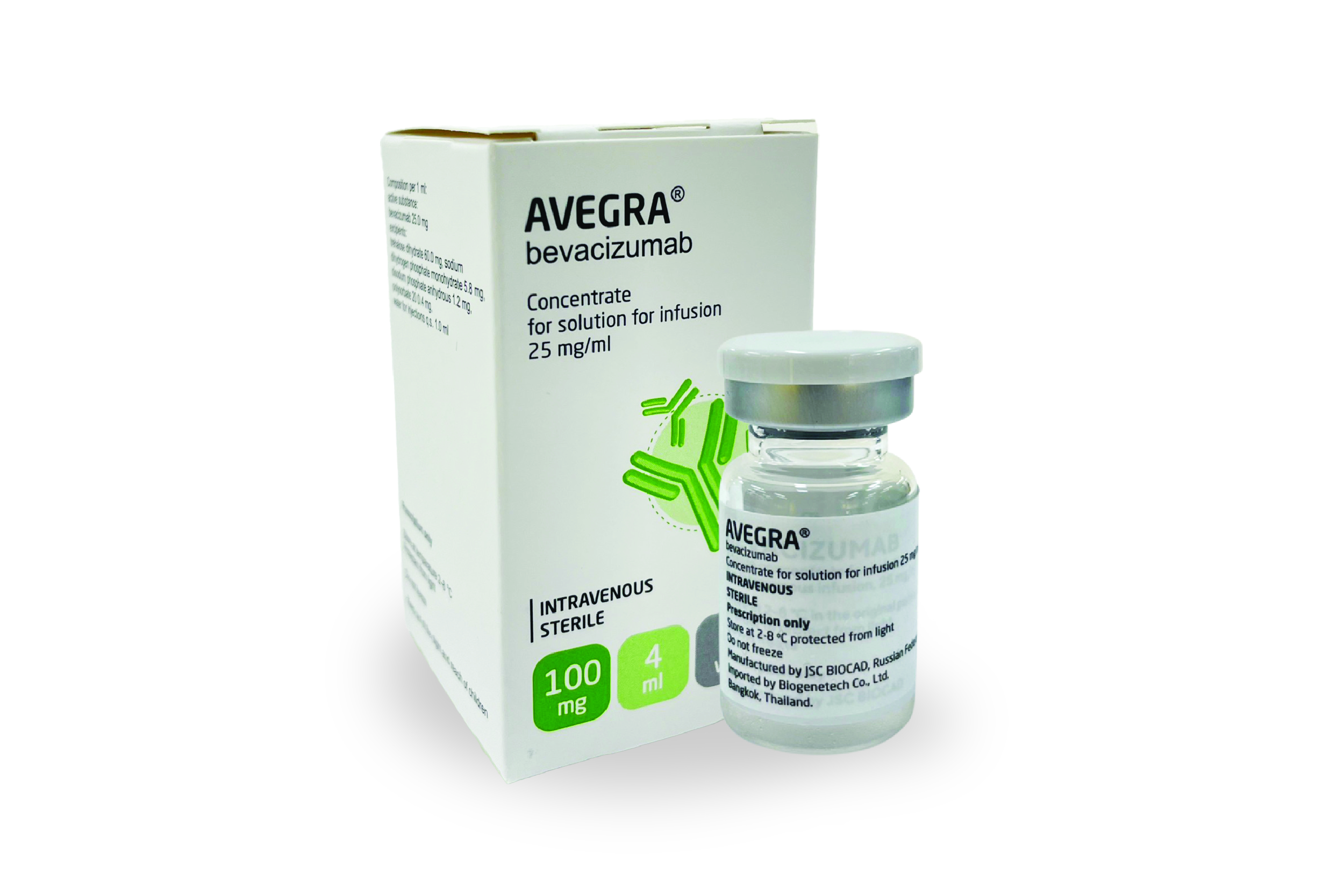
AVEGRA
(Bevacizumab)
Anti-VEGF Recombinant Monoclonal Antibody
Avegra®
Metastatic carcinoma of the colon or rectum (mCRC)
• Avegra®, in combination with a fluoropyrimidine-based chemotherapy is indicated for treatment of adult patients with metastatic carcinoma of the colon or rectum.
Non-small cell lung cancer other than predominantly squamous cell history (NSCLC)
First-line treatment of non-squamous NSCLC in combination with platinum-based chemotherapy
• Avegra® is administered for adult patients with unresectable advanced, metastatic or recurrent non-small cell lung cancer other than predominantly squamous cell histology.
First-line treatment of non-squamous NSCLC with EGFR activating mutations in combination with erlotinib
• Avegra® in combination with erlotinib, for adult patients with unresectable advanced, metastatic or recurrent non-squamous non-small cell lung cancer with
Epidermal Growth Factor Receptor (EGFR) activating mutations.
Epithelial ovarian, fallopian tube, or primary peritoneal cancer
Front-line treatment:
• Avegra® in combination with carboplatin and paclitaxel for adult patients with advanced (International Federation of Gynecology and Obstetrics (FIGO) stage IIIB,
IIIC and IV) epithelial ovarian, fallopian tube, or primary peritoneal cancer.
Treatment of platinum-sensitive recurrent disease:
• Avegra® in combination with carboplatin and gemcitabine for adult patients, who have not received prior therapy with bevacizumab or other VEGR inhibitors or VEGR receptor-targeted agents, for 6 cycles and up to 10 cycles follow by continued use of Avegra as single agent until disease progression.
Treatment of platinum-resistant recurrent disease:
• Avegra® in combination with paclitaxel, topotecan or pegylated liposomal doxorubicin indicated for the treatment of adult patients with platinum-resistant
recurrent epithelial ovarian, fallopian tube, or primary peritoneal cancer who received no more than two prior chemotherapy regimens and who have not received
prior therapy with bevacizumab or other VEGF inhibitors or VEGF receptor-targeted agents.
Carcinoma of the cervix
• Avegra® in combination with paclitaxel+cisplatin or alternatively, paclitaxel and topotecan in patients who cannot receive platinum therapy, is indicated for the treatment of adult patients with persistant, recurrent, or metastatic carcinoma of the cervix.
Renal cell cancer
• Avegra® in combination with interferon alfa-2a is indicated for first line treatment of adult patients with advanced and/or metastatic renal cell cancer.
Glioblastoma
• As monotherapy.
ใช้เฉพาะโรงพยาบาล
ความถูกต้องของโฆษณานี้ เป็นความรับผิดชอบของผู้โฆษณา มิได้ดำเนินการโดยสำนักงานคณะกรรมการอาหารและยา
เลขทะเบียน 1C 2/66 (NBS)
โปรดอ่านรายละเอียดเพิ่มเติมในเอกสารกำกับยา
ฆศ.2-1062/2566